Abstract
Objective: This study aimed to compare the short-term (three-month) effectiveness of three smoking cessation therapies, bupropion, nicotine patch, and cytisine, and to retrospectively evaluate the impact of cytisine therapy on craving reduction and its side effect profile under routine clinical practice conditions.
Methods: A total of 565 individuals who presented to the Smoking Cessation Outpatient Clinic between February 2024 and January 2025 and completed treatment with either cytisine, bupropion, or nicotine patch were included in the study. Demographic characteristics, chronic disease status, Fagerström Test for Nicotine Dependence (FTND) scores, and smoking cessation outcomes were assessed to compare treatment success across the three therapies. Among patients treated with cytisine, early craving reduction and reported side effects during the first week were recorded and analyzed in relation to cessation outcomes. Statistical significance was set at p<0.05.
Results: The mean age of participants was 41.28±12.33 years, and the mean FTND score was 6.18±2.32. Of the patients, 252 (44.7%) received cytisine, 135 (23.8%) received bupropion, and 178 (31.5%) received nicotine patch therapy. The smoking cessation rate was 61.5% in the cytisine group, 16.3% in the bupropion group, and 11.8% in the nicotine patch group. The cessation success rate in the cytisine group was significantly higher than in the other treatment groups (p<0.001). Among those who received cytisine, 161 patients (82.5%) reported a reduction in craving within the first five days of treatment. The overall side effect rate was 25%, with nausea and vomiting (22.7%) and headache (22.7%) being the most commonly reported adverse effects. Logistic regression analysis revealed a strong association between early craving reduction and successful smoking cessation among cytisine users (OR=25.79, p<0.001).
Conclusion: Cytisine was associated with higher cessation rates compared to other treatments. It also appeared to reduce cravings in the early phase and had a relatively low side effect profile. Craving reduction during the first week emerged as an important predictor of success. These findings suggest that cytisine may be an effective and well-tolerated option in primary care. However, further prospective studies are needed to evaluate its comparative effectiveness more comprehensively.
Keywords: smoking, nicotine, dependency, cytisine, bupropion, nicotine patch
Introduction
Tobacco use remains one of the leading preventable causes of death worldwide, contributing significantly to morbidity and mortality through its detrimental effects on the cardiovascular, respiratory, and metabolic systems.[1] Cigarette smoking is highly addictive due to the presence of nicotine, which is rapidly absorbed through the oral mucosa and alveoli, reaching the central nervous system and stimulating nicotinic acetylcholine receptors. This stimulation triggers the release of various neurotransmitters, most notably dopamine, forming the biochemical basis of nicotine dependence and withdrawal symptoms.[2]
Among the pharmacological treatment approaches developed for nicotine dependence, nicotine replacement therapies (NRT), varenicline, and bupropion are considered first-line medications, whereas cytisine is classified as a second-line agent. Although the efficacy of cytisine is comparable to that of first-line therapies, it is categorized separately due to its moderate level of evidence, limited number of countries in which it is licensed, and variability in dosing regimens. Nevertheless, all of these medications are supported by strong clinical recommendations and are considered viable options for smoking cessation treatment.[3] In Türkiye, while cytisine is not classified as a first-line agent in most international guidelines, it is widely available and provided free of charge in public smoking cessation clinics, contributing to its frequent use as a practical first-line option in local clinical practice.[4]
Systematic reviews and meta-analyses have demonstrated that cytisine exhibits a similar level of efficacy compared to NRT, bupropion, and varenicline.[5,6] Additionally, its greater cost-effectiveness relative to other pharmacological treatments has made it a valuable smoking cessation aid, particularly in low-resource settings.[7] However, most studies in the literature have been conducted under randomized controlled conditions, and real-world evidence remains limited. In particular, real-world data on cytisine use in Türkiye are scarce, which may limit generalizability. This study aims to address this gap by providing observational data from a Turkish clinical setting.
In this study, the short-term effects of pharmacological treatments on smoking cessation were compared among patients who presented to the smoking cessation outpatient clinic. In particular, the study aimed to retrospectively evaluate the effectiveness and side effect profile of cytisine therapy under routine clinical practice conditions.
Materials and Methods
This study retrospectively analyzed the data of individuals who presented to the smoking cessation outpatient clinic of a public hospital between February 2024 and January 2025.
During the specified period, a total of 1.112 patients presented to the clinic. Among them, 707 patients had attended at least one follow-up visit, had used the prescribed treatment, had known smoking cessation outcomes, and had complete electronic medical records. Of these, 565 patients who received one of the three most commonly prescribed pharmacological treatments (cytisine, bupropion, or nicotine patch) were included in the analysis (Figure 1). Specifically, 252 patients treated with cytisine, 135 with bupropion, and 178 with nicotine patch were analyzed. Patients who received combination therapy were excluded from the study. Medication use was confirmed based on patient self-reports during follow-up visits and cross-checked with electronic prescription and treatment adherence notes recorded by clinicians.
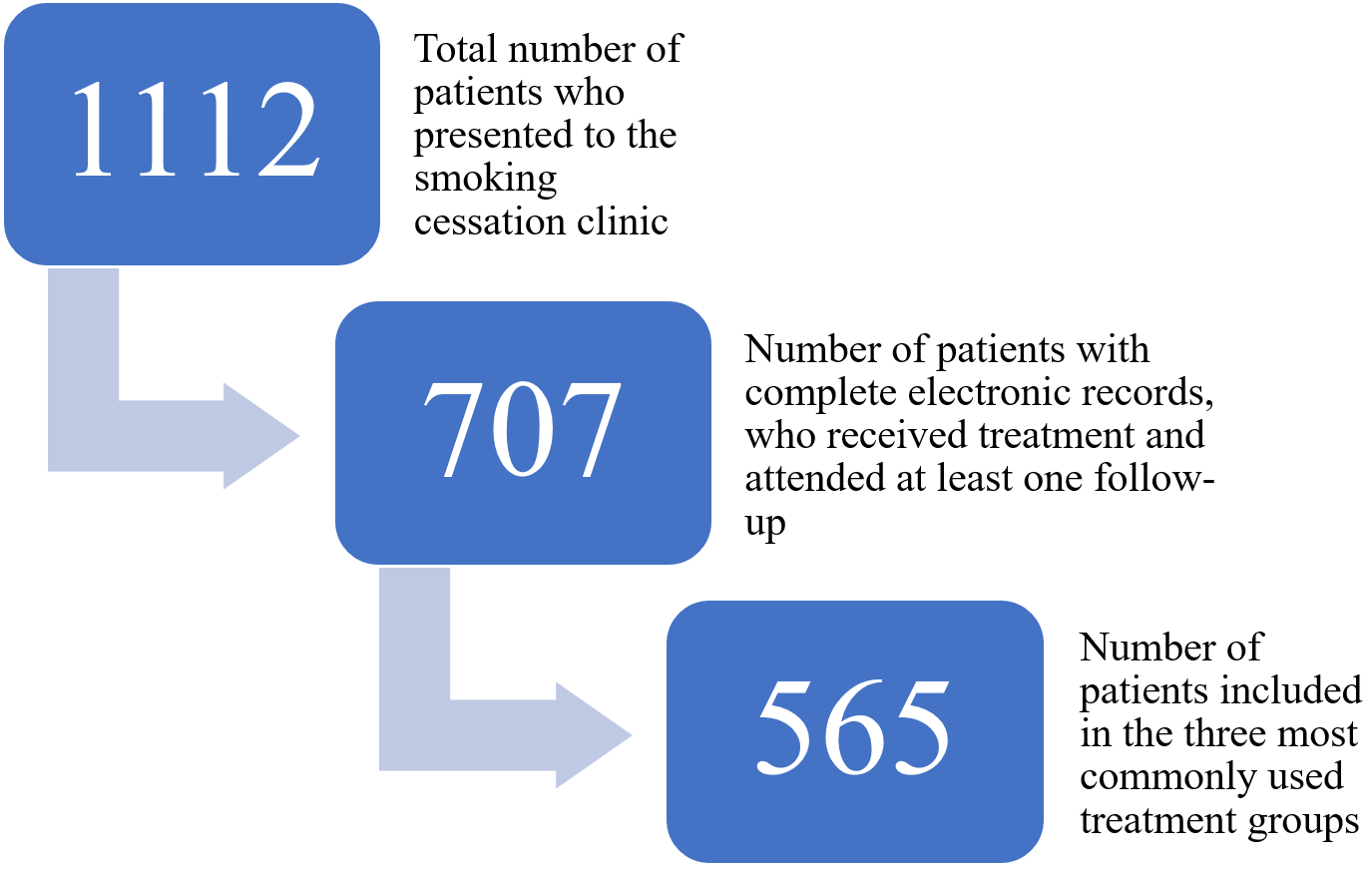
Demographic data such as age, sex, and educational level, as well as clinical information including the presence of chronic disease, Fagerström Test for Nicotine Dependence (FTND) scores, type of pharmacological treatment used, and reported medication side effects (for patients treated with cytisine), were obtained from electronic medical records. These data were retrieved from the hospital’s electronic medical record system, where detailed reports are routinely documented during patient visits.
Each patient’s smoking cessation status within the first three months was assessed through the electronic health records. According to the clinic’s standard follow-up protocol, patients were classified based on self-reported outcomes: those who declared abstinence from smoking were considered “successful,” those who did not quit at any point during treatment were labeled “unsuccessful,” and patients who resumed smoking within 3 months after initial cessation were categorized as having ‘’relapsed.’’ These classifications were based on patient statements recorded during follow-up visits. Objective biochemical verification methods (e.g., exhaled CO or cotinine testing) were not routinely employed due to limitations in clinical practice and resource constraints.
Subsequently, 252 patients who received cytisine and attended at least one follow-up visit were evaluated. This subgroup was examined in greater detail to assess the clinical effectiveness and side effect profile of cytisine therapy.
The standard cytisine regimen used in this study consisted of 1.5 mg tablets administered as follows: six tablets per day (one tablet every two hours) for the first three days (days 1–3); five tablets per day on days 4–12; four tablets per day on days 13–16; three tablets per day on days 17–20; and two tablets per day on days 21–25. The target quit date was set as Day 5 of treatment.[8]
During the initial follow-up visit conducted between Days 5 and 7 following the initiation of treatment, patients were assessed for reductions in craving to smoke, adherence to the treatment regimen, and early-onset side effects. For patients who did not attend the first-week follow-up, adverse effects were inquired about at subsequent appointments and recorded based on patient statements. Smoking cessation status was primarily determined through these self-reports. Additionally, electronic medical records were reviewed to verify prescription issuance, medication use, and attendance.
The study was designed as a cross-sectional, descriptive, single-center, and retrospective analysis. Ethical approval was obtained from the Health Science University Sisli Hamidiye Etfal Training and Research Hospital Ethics Committee on 14.01.2025, with protocol number 4725.
Statistical Analysis: All statistical analyses were performed using IBM SPSS statistical software, version 25.0. Descriptive statistics were presented as frequencies and percentages for categorical variables, and as mean, standard deviation, minimum, and maximum for continuous variables. Differences in proportions between independent groups were assessed using the Chi-square test. For continuous variables with a normal distribution, the Independent Samples T-test was used for two-group comparisons; for non-normally distributed variables, the Mann-Whitney U test was applied. The Kruskal-Wallis H test was used for comparisons among three independent groups when normality assumptions were not met, and one-way ANOVA was used when those assumptions were satisfied. The Kolmogorov-Smirnov test was employed to assess the normality of distribution. When normality was confirmed, Bonferroni post-hoc analysis was conducted to evaluate differences between groups.
To determine factors associated with smoking cessation, multivariate logistic regression analysis was performed. The bootstrap method was applied to enhance the reliability of the model and support parameter estimation. Odds ratios (ORs) and 95% confidence intervals (CIs) were reported. A p-value of <0.05 was considered statistically significant.
Results
The mean age of the 565 patients included in the analysis was 41.28±12.33 years (range: 18–75). The mean duration of smoking exposure was 18.21±26.32 years (range: 1–150), and the mean FTND score was 6.18±2.32 (range: 0–10). Of the participants, 213 (37.7%) were female, and 326 (57.7%) had a university-level education or higher. A total of 203 participants (35.9%) had at least one chronic disease.
Among the pharmacological treatment groups analyzed, 252 patients (44.7%) were treated with cytisine, 135 (23.8%) with bupropion, and 178 (31.5%) with nicotine patch therapy.
The mean FTND scores were 6.23±2.22 (range: 0–10) in the cytisine group, 6.39±2.36 in the bupropion group, and 5.97±2.42 in the NRT group. No statistically significant differences were found in FTND scores across the treatment groups (p>0.05, H=2.992).
When examining early-phase smoking cessation outcomes among all participants, 274 individuals (48.5%) were classified as unsuccessful, 198 (35.0%) as successful and 93 (16.5%) as relapsed. A statistically significant association was found between cessation outcomes and the type of treatment received (p<0.001). The smoking cessation rates were 61.5% (n=155) in the cytisine group, 16.3% (n=22) in the bupropion group, and 11.8% (n=21) in the nicotine patch group (Table 1).
| Table 1. Association between treatment type and smoking cessation status among all participants | ||||||
| Treatment Type |
|
|
|
|
|
|
| Cytisine | n |
|
|
|
|
|
| % of all treatments |
|
|
|
|
||
| % within cessation status |
|
|
|
|
||
| Bupropion | n |
|
|
|
|
|
| % of all treatments |
|
|
|
|
||
| % within cessation status |
|
|
|
|
||
| Nicotine patch | n |
|
|
|
|
|
| % of all treatments |
|
|
|
|
||
| % within cessation status |
|
|
|
|
||
| Total | n |
|
|
|
|
|
| % of all treatments |
|
|
|
|
||
| % within cessation status |
|
|
|
|
||
To determine which treatment groups accounted for the observed statistically significant difference, pairwise comparisons were performed. To control for the risk of type I error due to multiple comparisons, the Bonferroni correction was applied. Post hoc analysis revealed that the cytisine group differed significantly in terms of cessation success rates when compared to both the bupropion group (p<0.001) and the NRT group (p<0.001). However, no statistically significant difference was found between the bupropion and NRT groups (p>0.05).
Among the 252 patients who received cytisine, the mean age was 36.91±10.15 years (range: 18–64), and the mean FTND score was 6.23±2.22 (range: 0–10). Of these, 79 participants (31.3%) were female, and 114 (45.2%) had an education level of high school or below. A total of 201 individuals (79.8%) were employed, and 44 (17.5%) had at least one chronic disease.
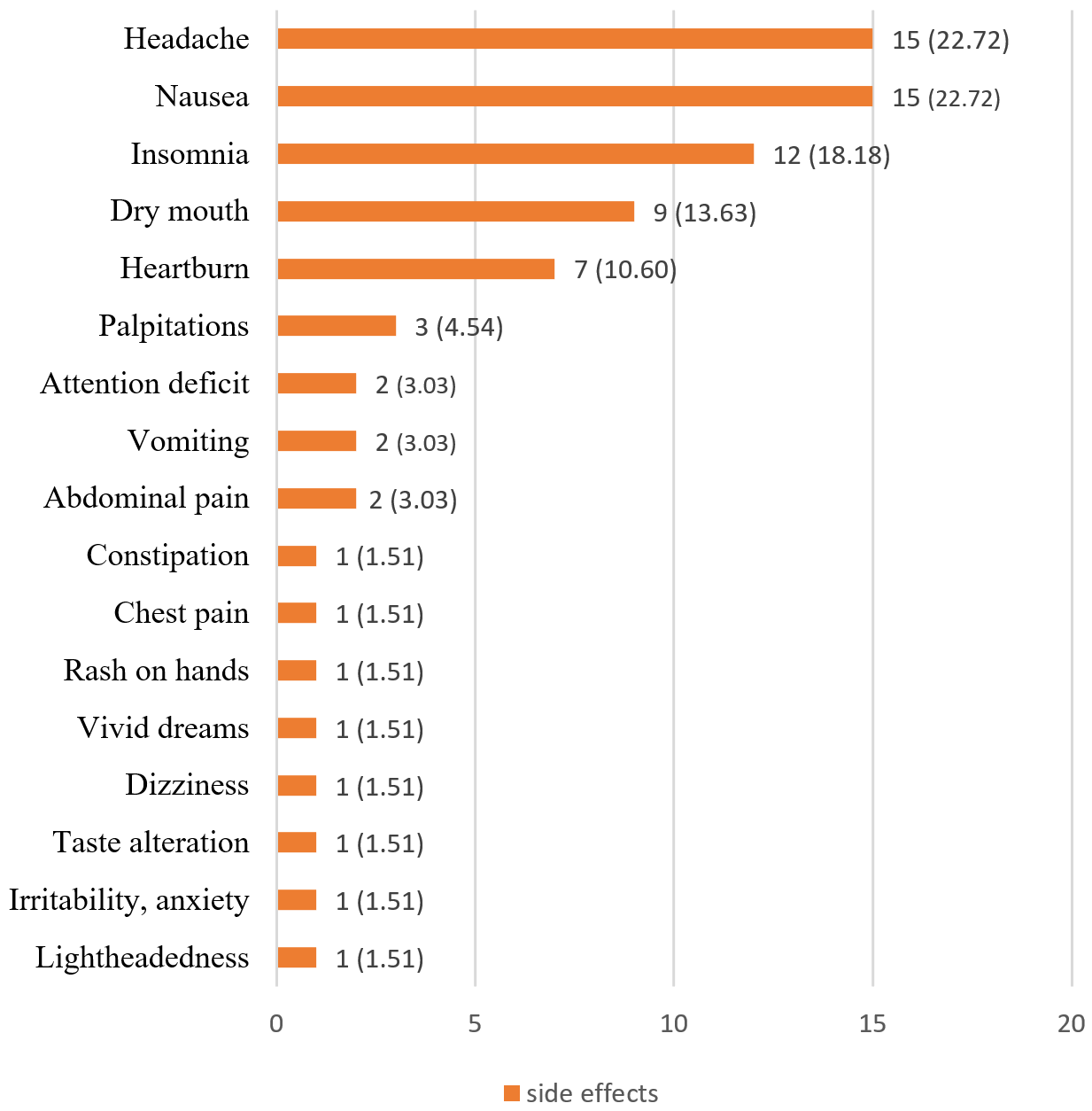
Regarding adverse effects, no side effects were reported in 191 participants (75.8%), while 61 participants (24.2%) reported at least one side effect. The most frequently reported side effects were nausea and vomiting (n=15, 22.7%), headache (n=15, 22.7%), and insomnia (n=12, 18.18%) (Figure 2). Treatment was discontinued in five participants (1.9%) due to side effects: in three cases due to nausea and vomiting, in one case due to nausea alone, and in one case due to severe insomnia lasting for three days in conjunction with nausea.
Among the 252 patients who received cytisine, 155 individuals (61.5%) successfully quit smoking, 68 individuals (27%) continued smoking, and 29 individuals (11.5%) experienced a relapse.
As presented in Table 2, no statistically significant relationship was found between smoking cessation status and age, sex, FTND score, employment status, educational level, presence of chronic disease, or adverse effect status (p>0.05). A statistically significant association was identified between smoking cessation status and reduction in craving (p<0.001). Further analysis revealed that the proportion of individuals who reported a reduction in craving was significantly higher in the group that successfully quit smoking compared to both those who did not quit and those who relapsed.
|
a ANOVA, b Mann-Whitney U, c Kruskal-Wallis H, d Independent t-test, e ANOVA post-hoc Bonferroni. * Craving reduction analysis was limited to 190 patients who attended the first-week follow-up visit. Patients who missed this visit (n=62) were excluded due to incomplete early-phase craving data. |
||||||||
| Table 2. Association of smoking cessation status and adverse effects with sociodemographic characteristics, FTND scores, and craving reduction in patients treated with cytisine (n=252) | ||||||||
| Unsuccessful1 | Successful2 | Relapsed3 | p | No Side Effects | Side Effects | p-value | ||
| Age (mean±SD, min–max) |
37.16±11.05 (18-64) |
37.48±9.92 (18-61) |
33.72±9.01 (19-54) |
0.187a | 37.06±10.16 (18-64) |
36.70±10.35 (18-58) |
0.731b | |
| FTND (mean±SD, min–max) |
6.32±2.20 (0-10) |
6.15±2.27 (1-10) |
6.38±2.04 (2-10) |
0.802c | 6.28±2.30 (0-10) |
6.06±1.96 (1-9) |
0.683d | |
| Sex, n (%) | Female | 22 (32.4) | 49 (31.6) | 8 (27.6) | 0.892 | 54 (28.3) | 25 (41.0) | 0.062 |
| Male | 46 (67.6) | 106 (68.4) | 21 (72.4) | 137 (71.7) | 36 (59.6) | |||
| Employment Status, n (%) | Unemployed | 17 (25.0) | 28 (18.1) | 6 (20.7) | 0.493 | 34 (17.8) | 17 (27.9) | 0.088 |
| Employed | 51 (75.0) | 127 (81.9) | 23 (79.3) | 157 (82.2) | 44 (72.1) | |||
| Education Level, n (%) | High school or below | 39 (57.4) | 63 (40.6) | 12 (41.4) | 0.063 | 84 (44.0) | 30 (49.2) | 0.477 |
| University or above | 29 (42.6) | 92 (59.4) | 17 (58.6) | 107 (56.0) | 31 (50.8) | |||
| Chronic Disease, n (%) | No | 55 (80.9) | 126 (81.3) | 27 (93.1) | 0.280 | 156 (81.7) | 52 (85.2) | 0.522 |
| Yes | 13 (19.1) | 29 (18.7) | 2 (6.9) | 35 (18.3) | 9 (14.8) | |||
| Side effects, n (%) | No | 55 (80.9) | 112 (72.3) | 24 (82.8) | 0.249 | - | ||
| Yes | 13 (19.1) | 43 (27.7) | 5 (17.2) | |||||
|
Craving Reduction (n=190) n (%)* |
No Reduction | 25 (83.3) | 2 (1.4) | 2 (12.5) |
<0.001 e2>1, e2>3 |
27 (19.1) | 2 (4.1) | 0.007 |
| Reduction | 5 (16.7) | 142 (98.6) | 14 (87.5) | 114 (80.9) | 47 (95.9) | |||
No statistically significant relationship was observed between the occurrence of side effects and age, sex, FTND score, educational status, employment, or presence of chronic disease (p>0.05). However, a statistically significant association was found between the presence of side effects and reduction in craving (p=0.007). The rate of reporting side effects was higher among individuals who experienced a reduction in craving compared to those who did not.
Sixty-two patients who did not attend the first-week follow-up were excluded from the craving reduction analysis. Among the remaining 190 patients, 161 (84.7%) reported a reduction in craving, while 29 (15.3%) did not. No statistically significant associations were found between craving reduction and age, sex, employment status, income level, or presence of chronic disease (p>0.05). However, a statistically significant association was observed between craving reduction and educational level (p=0.030). Among those who did not experience a reduction in craving, 62.1% (n=18) had a high school education or below, whereas among those who did experience a reduction in craving, 59.6% (n=96) completed university or higher education.
As presented in Table 3, a standard logistic regression analysis was conducted to identify the factors associated with smoking cessation in patients treated with cytisine. To enhance the reliability of parameter estimates and support the calculation of confidence intervals, a bootstrap method was applied.
| Table 3. Multivariate logistic regression analysis of the association between craving reduction, adverse effects, and smoking cessation in patients treated with cytisine | |||
|
|
|||
| Variables |
|
|
|
| Craving reduction |
|
|
|
| Adverse effects |
|
|
|
| Nagelkerke R2= 0.508 -2 Log likelihood=131.37 | |||
According to the model results, the variable reduction in craving was strongly and significantly associated with smoking cessation (OR=25.79, 95% CI=[13.12–47.02], p<0.001). This finding indicated that individuals who reported a reduction in craving had approximately 26 times higher odds of successfully quitting smoking compared to those who did not report such a reduction. In contrast, the variable adverse effects was not significantly associated with smoking cessation (p=0.492). The overall model fit was acceptable (Nagelkerke R²=0.508, −2 Log Likelihood=131.377).
Discussion
In this study, higher smoking cessation rates are observed among patients treated with cytisine compared to those receiving other pharmacological options within the same clinical setting. These findings indicate that cytisine may offer a potentially useful option for individuals attempting to quit smoking and could be considered as an alternative in primary care practice. The observed reduction in craving during the early phase of treatment, along with a relatively low rate of reported side effects, may support the tolerability and potential clinical utility of cytisine.Given its wide availability and cost-free access in public smoking cessation clinics in Türkiye, cytisine is frequently used as a first-line treatment in routine clinical practice, despite being considered a second-line agent in international guidelines.[3,4]
In this study, 61.5% of patients who received cytisine are found to have quit smoking in the early phase of treatment. The corresponding rates are 16.3% for bupropion and 11.8% for NRT. The cessation rate observed in the NRT group is consistent with the early-term (within the first six months) success rates reported in the literature, which typically range from 8% to 25%. This broad variability may be influenced by the type of NRT formulation used, patient adherence to treatment, and the quality of behavioral support provided.[9]
For bupropion, previous studies have reported end-of-treatment (typically 4–10 weeks) cessation rates ranging from 25% to 41% in the general adult population, although lower rates (around 11%) have also been documented in specific subgroups. The 16.3% success rate observed in our study lies near the lower end of this range and may be explained by individual variability, level of nicotine dependence, and adherence to treatment.[10-12]
Although the cessation rates for NRT and bupropion observed in our study fall within the lower end of ranges reported in the literature, several factors may contribute to these modest outcomes. During the study period, NRT and bupropion are not provided free of charge in our setting, except for cytisine, and the financial burden may limit patients’ ability to maintain the recommended treatment duration.[13,14] The required minimum treatment periods—three months for NRT and two months for bupropion—may also reduce adherence.[15,16] For both treatments, side effects and perceived effectiveness could discourage continuation. High nicotine dependence and long-term heavy smoking histories, common in our patient group, are known to predict lower quit rates.[17] These factors together may explain the relatively low success rates for NRT and bupropion in our setting.
Recent systematic reviews evaluating the efficacy of cytisine demonstrate that it is significantly more effective than placebo, and that, based on indirect comparisons, its success rates appear to be comparable to or even higher than those achieved with bupropion.[18] Some comparative studies find higher cessation rates in the cytisine group compared to the bupropion group; however, these differences are not statistically significant.[19] The findings of the present study are also consistent with previous data, including a study conducted in Italy that reports a 57.2% cessation rate at one month, and another that reports a rate of 40%.[20,21] Furthermore, a systematic review indicates that short-term (first-month) cessation success rates with cytisine ranged from 40% to 60%, and that cytisine was more effective than both placebo and NRT.[21] In this context, the 61.5% cessation rate observed in our study aligns with previously reported values and provides additional support for the potential efficacy of cytisine.
Cytisine acts as a partial agonist at α4β2 nicotinic acetylcholine receptors, mimicking the effects of nicotine to some extent while simultaneously blocking these receptors. This dual action helps reduce nicotine cravings and suppresses its rewarding effects.[22] This mechanism has been shown to contribute to a marked reduction in the urge to smoke during the initial days of treatment.[8,19] Consistently, in our study, 84.7% of patients assessed within the first week after initiating cytisine therapy report a reduction in craving, which is identified as a strong predictor of cessation success. Previous studies also suggest that initiating cytisine treatment before the designated quit day may alleviate withdrawal symptoms, support motivational processes, and enhance cessation outcomes.[23,24] Therefore, the follow-up visit during the first week of treatment is of critical importance for evaluating adherence and early treatment response.
Although educational levels were not directly associated with smoking cessation success with cytisine in our study, it is noteworthy that the majority of individuals who report craving reduction within the first five days had a university-level education or higher (p=0.030). This may suggest a potential indirect association between educational level and cessation success, as craving reduction is found to be the strongest independent predictor of quitting in our sample (OR=25.79, p<0.001). Existing literature also indicates that individuals with higher educational attainment tend to achieve greater success in smoking cessation efforts, which may be attributed to factors such as greater health awareness, stronger motivation, better adherence to treatment, and more effective use of support services.[25-27] However, this relationship is not universally observed. Several studies report no significant link between education and cessation success, which in some cases is attributed to the self-selection of participants in cessation programmes, leading to an overrepresentation of highly educated individuals and a reduced variability in educational attainment, as well as difficulties in standardising education categories across different educational systems.[28,29] In this study, 24.2% of participants who received cytisine report experiencing at least one side effect. The most commonly reported adverse effects are headache (22.7%), nausea (22.7%), and insomnia (18.1%), the majority of which are mild in severity. According to the Turkish product information for cytisine, adverse effects that occur in at least 1 in 10 patients are classified as “very common,” while those occurring in 1 to 10 out of 100 patients are considered “common”.[30] The listed very common side effects include dry mouth, nausea, abdominal pain, irritability, sleep disturbances (such as insomnia and abnormal dreams), anxiety, dizziness, skin rash, headache, acid reflux, and vomiting. Common side effects include concentration difficulties, burning sensation on the tongue, and bradycardia. The common side effects identified in our study belong to the category of “very common” adverse events as defined in the official product information.
In the literature, an observational study conducted in Italy reports that mild adverse events such as headache, stomach discomfort, and sleep disturbances are frequently observed following a 40-day cytisine treatment regimen.[20] Similarly, a randomized controlled trial (RCT) in New Zealand finds that nausea, vomiting, and sleep disturbances are more frequently reported in the cytisine group compared to the NRT group, although these are generally mild to moderate in intensity.[19]
In our study, treatment discontinuation due to side effects occurrs in 1.9% (n=5) of participants. This rate is comparable to that reported in a 12-week RCT evaluating cytisine, where treatment discontinuation due to adverse events was 2.9%.[3] In another RCT, the rate of serious adverse events was 3.1%, with no significant difference compared to the placebo group.[31] These findings support the conclusion that cytisine has a favorable tolerability profile and that the incidence of serious adverse events is low.
Interestingly, in our study, the rate of side effect reporting was higher among participants who experience a reduction in craving compared to those who did not. While this specific association has not been directly described in the literature, it is plausible that a rapid decrease in craving reflects greater neurobiological sensitivity to cytisine. In such individuals, increased nicotinic receptor responsiveness may underlie both improved craving control and the occurrence of mild side effects such as nausea and headache.[21,32] Further research is needed to better elucidate the underlying mechanisms of this observation.This study has several limitations. The analysis relies on electronic medical records and patient self-reports to assess smoking cessation outcomes and medication adherence. Although electronic data are used to verify prescription and follow-up attendance, these sources may not fully reflect actual medication use or abstinence status. The lack of biochemical verification is a notable limitation and may lead to overestimation of cessation success. Furthermore, it is not possible to objectively confirm whether all patients use the medications fully and as prescribed. Smoking cessation success is evaluated based on patient status during the first three months, regardless of treatment duration. While this approach reflects real-world clinical practice and allows standardized comparison, it may overlook the impact of varying treatment durations and quit date timing across different pharmacological therapies. Additionally, craving reduction is assessed through patient self-report, which may introduce subjectivity into this outcome.
In this study, the short-term smoking cessation success rates of cytisine, bupropion, and NRT are compared. The cessation rate is found to be higher in the group treated with cytisine. In contrast, the success rates observed in the bupropion and NRT groups are closer to the lower limits of the ranges reported in the literature.
Cytisine stands out not only for its relatively high cessation rate but also for its capacity to significantly reduce the urge to smoke during the early phase of treatment and for its favorable side effect profile. These characteristics suggest that cytisine may be a supportive agent in enhancing treatment adherence and cessation outcomes. The mild nature of the reported adverse effects and the low rate of treatment discontinuation further support its tolerability. Reduction in craving observed during the first week may serve as an important predictor of cessation success and could play a critical role in guiding treatment decisions during this early period.
Cytisine may be considered a strong treatment option in primary care smoking cessation programs due to its efficacy and safety profile. However, to more robustly assess the generalizability of these findings and the long-term outcomes of treatment, further research is needed, particularly well-designed, multicenter, prospective, and comparative studies.
Ethical approval
This study has been approved by the Health Sciences University Sisli Hamidiye Etfal Training and Research Hospital Ethics Committee (approval date 14.01.2025, number 4725).
Source of funding
The authors declare the study received no funding.
Conflict of interest
The authors declare that there is no conflict of interest.
References
- Etter JF. Cytisine for smoking cessation: a literature review and a meta-analysis. Arch Intern Med. 2006;166(15):1553-1559. https://doi.org/10.1001/archinte.166.15.1553
- Benowitz NL. Clinical pharmacology of nicotine: implications for understanding, preventing, and treating tobacco addiction. Clin Pharmacol Ther. 2008;83(4):531-541. https://doi.org/10.1038/clpt.2008.3
- World Health Organization (WHO). WHO clinical treatment guideline for tobacco cessation in adults. Geneva: World Health Organization; 2024.
- T.C. Sağlık Bakanlığı, Halk Sağlığı Genel Müdürlüğü. Ülkemizdeki tütün kontrol çalışmaları. 2024. Available at: https://havanikoru.saglik.gov.tr/tuetuen-hakkinda/uelkemizdeki-tuetuen-kontrol-calismalari.html (Accessed on Feb 7, 2025).
- Ofori S, Lu C, Olasupo OO, et al. Cytisine for smoking cessation: a systematic review and meta-analysis. Drug Alcohol Depend. 2023;251:110936. https://doi.org/10.1016/j.drugalcdep.2023.110936
- Livingstone-Banks J, Fanshawe TR, Thomas KH, et al. Nicotine receptor partial agonists for smoking cessation. Cochrane Database Syst Rev. 2023;5(5):CD006103. https://doi.org/10.1002/14651858.CD006103.pub8
- Tutka P, Vinnikov D, Courtney RJ, Benowitz NL. Cytisine for nicotine addiction treatment: a review of pharmacology, therapeutics and an update of clinical trial evidence for smoking cessation. Addiction. 2019;114(11):1951-1969. https://doi.org/10.1111/add.14721
- West R, Zatonski W, Cedzynska M, et al. Placebo-controlled trial of cytisine for smoking cessation. N Engl J Med. 2011;365(13):1193-1200. https://doi.org/10.1056/NEJMoa1102035
- Carpenter MJ, Wahlquist AE, Dahne J, et al. Nicotine replacement therapy sampling for smoking cessation within primary care: results from a pragmatic cluster randomized clinical trial. Addiction. 2020;115(7):1358-1367. https://doi.org/10.1111/add.14953
- Kranzler HR, Washio Y, Zindel LR, et al. Placebo-controlled trial of bupropion for smoking cessation in pregnant women. Am J Obstet Gynecol MFM. 2021;3(6):100315. https://doi.org/10.1016/j.ajogmf.2021.100315
- Aubin HJ, Lebargy F, Berlin I, Bidaut-Mazel C, Chemali-Hudry J, Lagrue G. Efficacy of bupropion and predictors of successful outcome in a sample of French smokers: a randomized placebo-controlled trial. Addiction. 2004;99(9):1206-1218. https://doi.org/10.1111/j.1360-0443.2004.00814.x
- Lee AM, Jepson C, Hoffmann E, et al. CYP2B6 genotype alters abstinence rates in a bupropion smoking cessation trial. Biol Psychiatry. 2007;62(6):635-641. https://doi.org/10.1016/j.biopsych.2006.10.005
- Cihan FG, Savran N, et al. Nikotin bağımlılığında sitizin tedavisi [Cytisine treatment in nicotine addiction]. Jour Turk Fam Phy. 2025;16(2):124-136. https://doi.org/10.15511/tjtfp.25.00224
- Altman D, Clement F, Barnieh L, Manns B, Penz E. Cost effectiveness of universally funding smoking cessation pharmacotherapy. Can J Respir Crit Care Sleep Med. 2019;3(2):67-75. https://doi.org/10.1080/24745332.2018.1512840
- Claire R, Chamberlain C, Davey MA, et al. Pharmacological interventions for promoting smoking cessation during pregnancy. Cochrane Database Syst Rev. 2020;3(3):CD010078. https://doi.org/10.1002/14651858.CD010078.pub3
- Cahill K, Stevens S, Perera R, Lancaster T. Pharmacological interventions for smoking cessation: an overview and network meta-analysis. Cochrane Database Syst Rev. 2013;2013(5):CD009329. https://doi.org/10.1002/14651858.CD009329.pub2
- Karadoğan D, Önal Ö, Şahin DS, Kanbay Y, Alp S, Şahin Ü. Treatment adherence and short-term outcomes of smoking cessation outpatient clinic patients. Tob Induc Dis. 2018;16:38. https://doi.org/10.18332/tid/94212
- Livingstone-Banks J, Lindson N, Hartmann-Boyce J. Effects of interventions to combat tobacco addiction: cochrane update of 2021 to 2023 reviews. Addiction. 2024;119(12):2101-2115. https://doi.org/10.1111/add.16624
- Walker N, Howe C, Glover M, et al. Cytisine versus nicotine for smoking cessation. N Engl J Med. 2014;371(25):2353-2362. https://doi.org/10.1056/NEJMoa1407764
- Principe R, Tinghino B, Zagà V, et al. Use of cytisine for smoking cessation in Italian Centers for Tobacco Treatment. Eur Respir J. 2017;50(suppl 61):PA4476. https://doi.org/10.1183/1393003.congress-2017.PA4476
- Puljević C, Stjepanović D, Meciar I, et al. Systematic review and meta-analyses of cytisine to support tobacco cessation. Addiction. 2024;119(10):1713-1725. https://doi.org/10.1111/add.16592
- Rigotti NA, Benowitz NL, Prochaska J, et al. Cytisinicline for smoking cessation: a randomized clinical trial. JAMA. 2023;330(2):152-160. https://doi.org/10.1001/jama.2023.10042
- Ferguson SG, Walters JAE, Lu W, Wells GP, Schüz N. Examination of the mechanism of action of two pre-quit pharmacotherapies for smoking cessation. BMC Public Health. 2015;15:1268. https://doi.org/10.1186/s12889-015-2596-2
- Walker N, Smith B, Barnes J, et al. Cytisine versus varenicline for smoking cessation in New Zealand indigenous Māori: a randomized controlled trial. Addiction. 2021;116(10):2847-2858. https://doi.org/10.1111/add.15489
- Ruokolainen O, Härkänen T, Lahti J, Haukkala A, Heliövaara M, Rahkonen O. Association between educational level and smoking cessation in an 11-year follow-up study of a national health survey. Scand J Public Health. 2021;49(8):951-960. https://doi.org/10.1177/1403494821993721
- Assari S, Sheikhattari P. Social determinants of successful smoking cessation: an eight-year analysis of Population Assessment of Tobacco and Health (PATH) data. J Biomed Life Sci. 2024;4(2):60-70. https://doi.org/10.31586/jbls.2024.1070
- Goding Sauer A, Fedewa SA, Kim J, Jemal A, Westmaas JL. Educational attainment & quitting smoking: a structural equation model approach. Prev Med. 2018;116:32-39. https://doi.org/10.1016/j.ypmed.2018.08.031
- Monsó E, Campbell J, Tønnesen P, Gustavsson G, Morera J. Sociodemographic predictors of success in smoking intervention. Tob Control. 2001;10(2):165-169. https://doi.org/10.1136/tc.10.2.165
- Erbaş G, Şengezer T, Yıldırım U, Özkara A. Ankara’da bir kadın doğum hastanesine başvuran gebelerde sigara kullanımı ve sigara dumanından pasif etkilenme durumlarının araştırılması [Investigation of active and passive smoking in pregnant women applying to a maternity hospital in Ankara]. Konuralp Med J. 2020;12(2):261-269. https://doi.org/10.18521/ktd.653859
- Nobel İlaç. Nikitabs ilaç prospektüsü [Nikitabs prescribing information]. Available at: https://pdf.ilacprospektusu.com/21410-nikitabs-1-5-mg-film-kapli-tablet-kt.pdf (Accessed on Jun 24, 2025).
- Rungruanghiranya S, Tulatamakit S, Chittawatanarat K, et al. Efficacy and safety of cytisine versus nortriptyline for smoking cessation: a multicentre, randomized, double-blinded and placebo-controlled trial. Respirology. 2024;29(10):880-887. https://doi.org/10.1111/resp.14787
- Igari M, Alexander JC, Ji Y, Qi X, Papke RL, Bruijnzeel AW. Varenicline and cytisine diminish the dysphoric-like state associated with spontaneous nicotine withdrawal in rats. Neuropsychopharmacology. 2014;39(2):455-465. https://doi.org/10.1038/npp.2013.216
Copyright and license
Copyright © 2025 The Author(s). This is an open access article distributed under the Creative Commons Attribution License (CC BY), which permits unrestricted use, distribution, and reproduction in any medium or format, provided the original work is properly cited.










