Abstract
Bullous pemphigoid is the most common bullous chronic autoimmune skin disease. Recent studies suggest dipeptidyl peptidase-4 inhibitors used in the treatment of type-2 diabetes as possible predisposing agents of bullous pemphigoid. It is thought to be important for primary care physicians, who are the most common referral point of patients and where drug use is examined in detail, to keep in mind that drugs such as dipeptidyl-peptidase-4 inhibitors may cause bullous pemphigoid and to refer to dermatology for early treatment.
In this context, a 70-year-old female patient with a diagnosis of type 2 diabetes mellitus presented to our outpatient clinic with pruritic, erythematous bullous lesions diffusely distributed over the body, occurring one week after initiation of a vildagliptin-containing medication. There was no mucosal involvement. The patient initially received treatment with the presumptive diagnoses of allergy and scabies; however, as no improvement was observed, a detailed medical history was obtained. Based on the clinical findings, drug-induced bullous pemphigoid was suspected, and the vildagliptin-containing medication was discontinued. The patient was subsequently referred to the dermatology department. Histopathological examination and direct immunofluorescence findings were consistent with bullous pemphigoid. Following drug withdrawal and a short course of oral corticosteroid therapy, a marked improvement of the lesions was achieved.
With this case, we aimed to draw attention to the approach of bullous pemphigoid disease developing as a result of vildagliptin use in a patient with type-2 diabetes who presented to primary care and to increase awareness on this issue.
Keywords: bullous pemphigoid, dipeptidyl peptidase-4 inhibitors, primary care
Introduction
Bullous pemphigoid (BP), characterized by severe pruritus, tense bullae and edematous erythema, is the most common autoimmune bullous disease and mainly affects the elderly.[1,2] Drug-associated BP, which accounts for up to 10% of pemphigus cases, is a term that indicates clinical, histologic, or immunopathologic features of the idiopathic form of bullous pemphigoid associated with systemic or topical administration of certain drugs.[3]
In recent years, the incidence has increased in association with different medications, including diuretics, beta-blockers and antibiotics (Table 1), and has recently been associated with the use of dipeptidyl peptidase-4 (DPP4) inhibitors, which are recommended in combination or as monotherapy for the treatment of type-2 diabetes.[4] In DPP4 inhibitor-associated BP, lesions usually begin several months to years after the start of use of a DPP-4 inhibitor. Available data describe a relatively higher risk with vildagliptin. This is followed by linagliptin, sitagliptin and saxagliptin.[5]
| Table 1. Medications implicated in drug-associated bullous pemphigoid | ||
| Likely associationa | Probable associationb | Uncertain associationc |
| Alogliptin | Actinomycin-D | Aldesleukin (IL-2) |
| Anagliptin | Adalimumab | Amantadine |
| Aspirin | Amoxicillin | Amlodipine |
| Biostim® | Ampicillin | Anthralin (dithranol) |
| D-Penicillamine | Arsenic | Azapropazone |
| Enalapril | Atezolizumab | Captopril |
| Erlotinib | Bumetanide | Coal tar |
| Etanercept | Celecoxib | Complementary medicines |
| Everolimus | Cephalexin | Dabrafenib |
| Furosemide | Chloroquine | Doxepin |
| Ibuprofen | Ciprofloxacin | Enoxaparin |
| Levofloxacin | Diclofenac | Escitalopram |
| Linagliptin | Dorzolamide | Fluorouracil |
| Nivolumab | Durvalumab | Flupenthixol |
| Pembrolizumab | Efalizumab | Galantamine hydrobromide |
| Phenacetin | Fluoxetine | Herpes zoster vaccine |
| Psoralens with UVA | Gabapentin | Influenza vaccine |
| Rifampicin | Griseofulvin | Iodide |
| Serratiopeptidase | Hepatitis B vaccine | Levetiracetam |
| Sirolimus | Hexavalent combined | Mesalazine |
| Sitagliptin | vaccine | Nadolol |
| Teneligliptin | Hydrochlorothiazide | Nifedipine |
| Tetanus toxoid | Infliximab | Novoscabin (benzyl |
| Tiobutarit | Ipilimumab | benzoate) |
| Vildagliptin | Lisinopril | Omeprazole |
| Losartan | Placental extracts | |
| Mefenamic acid | Photodynamic therapy | |
| Metamizole | Risperidone | |
| Metronidazole | Rotavirus vaccine | |
| Penicillin | Sulfonamide | |
| Rosuvastatin | Swine flu vaccine | |
| Spironolactone | Timolol | |
| Sulfasalazine | Valsartan | |
| Terbinafine | ||
| Ustekinumab | ||
Drug-associated BP also tends to occur without severe urticaria or erythema at the base of the lesions, unlike inflammatory bullous pemphigoid. Stopping the DPP-4 inhibitor results in complete resolution of symptoms in about one third of patients. Patients who are still symptomatic should be referred to a dermatologist for treatment with corticosteroids or tetracyclines.[6]
When the drug is not withdrawn after diagnosis, the response is mild or reappears after a short time.[5,7]
To prevent recurrence, the addition of systemic corticosteroids is recommended. In moderate to severe cases, an initial oral corticosteroid dose of 1 mg/kg/day is advised, while lower doses of 0.2–0.5 mg/kg/day are administered in mild cases.[2,8] The dose is gradually reduced in the absence of new lesions and when the disease stabilizes. After one month of treatment, improvement is usually marked. It may persist for up to several months after drug discontinuation and is characterized by rare relapses.[9]
With this case report, we aimed to draw attention to drug-associated bullous pemphigoid disease through a case of bullous pemphigoid secondary to vildagliptin use and to draw attention to the importance of recognition by primary care physicians and appropriate referral for early treatment.
Case Presentation
Patient information
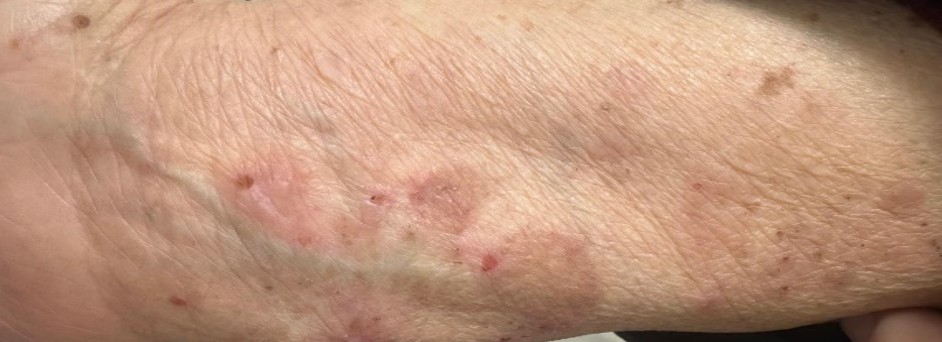
A 70-year-old female patient with a history of hypertension and type 2 diabetes mellitus (DM) presented with erythematous, pruritic, tense bullae that initially appeared on the arms and legs and progressively spread over the entire body. No mucosal involvement was detected (Figure 1).
Medical history
The patient reported that the skin lesions appeared after she restarted her oral antidiabetic therapy containing vildagliptin, which she had been using irregularly for approximately two years. The eruptions began one week after reinitiating the medication.
She had previously visited the emergency department twice for these complaints — initially diagnosed and treated for allergy, and later for scabies — but no clinical improvement was observed. The patient subsequently presented to our family medicine outpatient clinic, where a detailed history was obtained and her medications were reviewed.
Current medications
Silazapril + Hydrochlorothiazide, Pitavastatin, Vildagliptin, Propranolol.
Preliminary diagnosis and management
Considering the possibility of bullous pemphigoid induced by vildagliptin, the medication was discontinued, and the patient was referred to the dermatology outpatient clinic. Following consultation with the internal medicine department, insulin therapy was initiated in place of the discontinued oral antidiabetic agent.
Dermatological evaluation and diagnosis
The dermatology specialist also suspected drug-induced bullous pemphigoid and referred the patient to a tertiary center for biopsy.
Biopsy findings
- Histopathology: Subepidermal bullae formation, infiltration of polymorphonuclear leukocytes within the bullae, perivascular mononuclear inflammatory cell infiltration in the dermis, and orthokeratosis in the epidermis.
- Direct Immunofluorescence (DIF): Linear positivity for C3 and IgG along the basement membrane zone; negative for IgA, IgM, and fibrinogen.
- Diagnosis: Findings consistent with bullous pemphigoid.
Treatment and clinical course
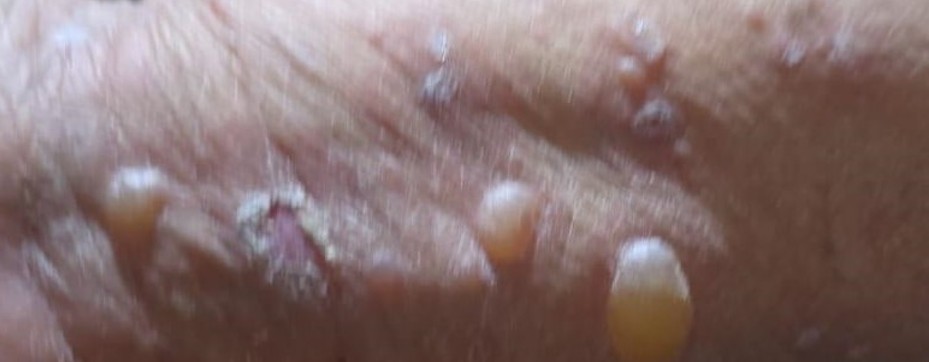
Following the discontinuation of the vildagliptin-containing medication, a marked regression of the lesions was observed. The dermatology department initiated a 14-day oral corticosteroid regimen: First 5 days: 16 mg twice Daily, Subsequent 9 days: 16 mg once Daily. Significant clinical improvement of the lesions was achieved after treatment (Figure 2).
Discussion
Bullous pemphigoid is an acquired autoimmune disease characterized by subepidermal blistering and mainly affects the elderly. The pathogenesis of the condition has not yet been fully elucidated, but it is widely accepted that there may be a strong correlation with various drugs.[10]
To date, more than 60 drugs have been reported to induce BP, including some antibiotics, anti-hypertensive drugs, anti-TNF-α drugs and vaccines.[11] Among all classes of drugs, robust evidence suggests that DPP-4 inhibitor prior use carries the highest risk for BP. In a meta-analysis by Kridin and Cohen, it was reported that the risk of developing BP increased 3.2-fold following DPP-4 inhibitor administration, and this risk was found to have the highest association with vildagliptin among DPP-4 inhibitors.[12],[13]
Currently, there is a rapidly increasing volume of publications on DPP-4 inhibitor-associated BP, which means that it is now an important topic in this field.[11]
In addition, the present cases demonstrated the difficulty in the diagnosis of BP associated with DPP-4 inhibitors. This is due to the fact that drug-induced BP presents a diverse clinical picture in terms of agents and cutaneous inflammations, especially latency duration. Because of these features, clinicians should be fully aware of the potential risk. The current cases demonstrate the importance of early diagnosis and prompt withdrawal of agents to prevent exacerbation of skin symptoms. Since complete remission takes at least 2 weeks to achieve, appropriate withdrawal of agents is required for remission of BP in suspected cases.[14]
Conclusion
Drug-induced BP is difficult to diagnose, unlike its idiopathic counterpart. This is because in both cases the clinical picture and histopathologic findings have only subtle differences. Patients presenting with BP and receiving multiple therapies should always be suspected to have a drug-induced variant of the condition. This possibility should be considered as most patients respond rapidly to treatment and do not relapse after discontinuation of the suspected drug.
Primary care physicians have great importance in identifying drug-induced BP cases early and organizing the treatment of patients by providing a multidisciplinary approach with the relevant units.
Therefore, we suggest that the diagnosis of drug-induced BP should be kept in mind among elderly diabetics presenting to primary care with initial signs and symptoms of BP.
Ethical approval
Informed consent form was obtained from the patient for this case.
Source of funding
The authors declare the study received no funding.
Conflict of interest
The authors declare that there is no conflict of interest.
References
- Miyamoto D, Santi CG, Aoki V, Maruta CW. Bullous pemphigoid. An Bras Dermatol. 2019;94(2):133-146. https://doi.org/10.1590/abd1806-4841.20199007
- Di Lernia V, Casanova DM, Goldust M, Ricci C. Pemphigus vulgaris and bullous pemphigoid: update on diagnosis and treatment. Dermatol Pract Concept. 2020;10(3):e2020050. https://doi.org/10.5826/dpc.1003a50
- Verheyden MJ, Bilgic A, Murrell DF. A systematic review of drug-induced pemphigoid. Acta Derm Venereol. 2020;100(15):adv00224. https://doi.org/10.2340/00015555-3457
- Molina-Guarneros JA, Sainz-Gil M, Sanz-Fadrique R, et al. Bullous pemphigoid associated with the use of dipeptidil peptidase-4 inhibitors: analysis from studies based on pharmacovigilance databases. Int J Clin Pharm. 2020;42(2):713-720. https://doi.org/10.1007/s11096-020-01003-6
- Sun L, Wang C, Wu C, Zhou Y, Wang C. Analysis of the clinical characteristics of dipeptidyl peptidase-4 inhibitor-induced bullous pemphigoid. Ann Pharmacother. 2022;56(2):205-212. https://doi.org/10.1177/10600280211022722
- Kano Y, Kato M. Bullous pemphigoid associated with use of dipeptidyl peptidase-4 inhibitor. CMAJ. 2022;194(20):E705. https://doi.org/10.1503/cmaj.211933
- Prugnit-Rouanet M, Delaumenie S, Couraud A, Audevard D, Rouanet J, Bedane C. Gliptin-induced bullous pemphigoid: withdrawal of gliptin allows for faster control of the disease. Eur J Dermatol. 2022;32(3):368-372. https://doi.org/10.1684/ejd.2022.4270
- Fenne IJ, Askildsen Oftebro G, Vestergaard C, Frølunde AS, Bech R. Effect of early initiation of steroid-sparing drugs in patients with bullous pemphigoid. Front Immunol. 2023;14:1176284. https://doi.org/10.3389/fimmu.2023.1176284
- Kanahara SM, Agrawal A. Drug-induced bullous pemphigoid. J Gen Intern Med. 2016;31(11):1393-1394. https://doi.org/10.1007/s11606-016-3679-1
- Stavropoulos PG, Soura E, Antoniou C. Drug-induced pemphigoid: a review of the literature. J Eur Acad Dermatol Venereol. 2014;28(9):1133-1140. https://doi.org/10.1111/jdv.12366
- Tasanen K, Varpuluoma O, Nishie W. Dipeptidyl peptidase-4 inhibitor-associated bullous pemphigoid. Front Immunol. 2019;10:1238. https://doi.org/10.3389/fimmu.2019.01238
- Kridin K, Cohen AD. Dipeptidyl-peptidase IV inhibitor-associated bullous pemphigoid: a systematic review and meta-analysis. J Am Acad Dermatol. 2021;85(2):501-503. https://doi.org/10.1016/j.jaad.2018.09.048
- Shalmon D, Bar-Ilan E, Peled A, et al. Identification of risk factors for gliptin-associated bullous pemphigoid among diabetic patients. Acta Derm Venereol. 2024;104:adv26663. https://doi.org/10.2340/actadv.v104.26663
- Yoshiji S, Murakami T, Harashima SI, et al. Bullous pemphigoid associated with dipeptidyl peptidase-4 inhibitors: A report of five cases. J Diabetes Investig. 2018;9(2):445-447. https://doi.org/10.1111/jdi.12695
Copyright and license
Copyright © 2025 The Author(s). This is an open access article distributed under the Creative Commons Attribution License (CC BY), which permits unrestricted use, distribution, and reproduction in any medium or format, provided the original work is properly cited.